2024 Swiss TB Award
The SwissTB award has been given this year to Dr. Melanie Hannebelle, Dr. Richa Mishra, Dr. Vivek Thacker for their study published in Cell.



Tuberculosis (TB), a chronic respiratory infection, remains a top global health threat with approximately 10 million new cases and 1.4 million deaths annually. A striking visual characteristic of the causative virulent strain Mycobacterium tuberculosis (Mtb) is their ability to grow into serpentine, high-aspect ratio “cords” in detergent-free media, a feature identified 80 years ago. These geometries do not occur in typical biofilms and since cord formation is conserved across pathogenic Mycobacterium spp, it suggests an important role for the architecture itself in pathogenesis. Cording had been attributed to the properties of the surface lipids in the mycomembrane, although the biophysical mechanisms by which several generations of dividing bacteria could “hang together” and form cords had not been elucidated.
In our study, published in Cell in 2023, we used a unique combination of technologies to systematically unravel the role of the cord architecture per se in pathogenesis and virulence. Our lung-on-chip model allowed us a direct look at “first contact” of Mtb with host cells at the air-liquid interface, and we found that cord formation was prominent in early infection. Notably, through confocal imaging of thick lung tissue slices, we directly confirmed that cording also occurred early in infection in mouse models. We then leveraged experiments between the lung-on-chip, the C3HeB/FeJ mouse model that develops pathologies mimicking human TB, and direct comparisons between a wild-type and a cording-deficient Mtb mutant strain to delineate distinct roles for the cord architecture that followed a temporal sequence. Our results showed a mechanobiological role for cords in TB pathogenesis which contributed to the relative suppression of innate immune signaling in early infection with wild-type but not the cording-deficient Mtb. We found that “extracellular” cords released from dying cells interact with the surrounding host tissue to cause cell-type specific death while being able to grow in-between epithelial cells by breaking tight junctions due to the structural cohesion of the cord. This mechanobiological observation explained several aspects of pathology such as increased pulmonary inflammation, elevated Th17 responses, delayed dissemination to the spleen and a significantly higher number of bacilli contained within inflamed alveoli in infection with the cording-deficient mutant.
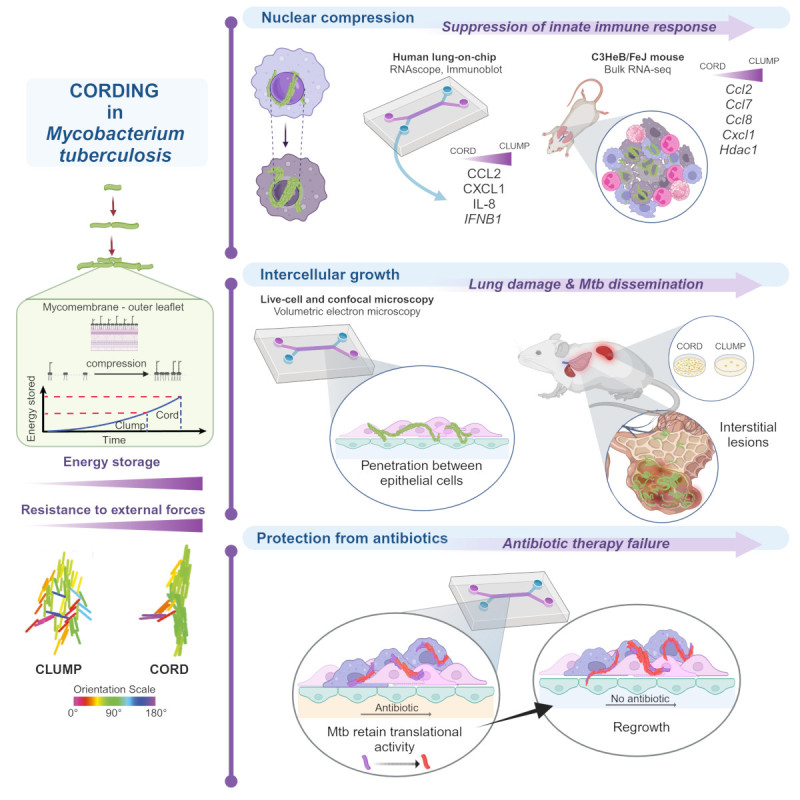
In order to delineate the biophysical mechanism of cord formation, we directly measured compressibility of lipids from the outermost later of “free lipids” in the Mtb cell wall to show that lipids from the cording-deficient mutant stored ca. four-fold less energy than wild-type bacteria when compressed. We developed a new agent-based model that specifically incorporated the energy stored in lipid compression as a tunable parameter, to demonstrate that an increased storage of energy in membranes of adjacent cells was sufficient to form cord architectures. Using the model, we showed that cords retain their structural integrity both in the presence of external noise as well as sudden external forces, whereas clump-like microcolonies that formed from bacteria with properties that mimic the cording-deficient mutant came apart when perturbed. We thus demonstrated, in silico, the ability of cords to exert forces on their surroundings, in concordance with the observations in vitro and in vivo.
Finally, we identified a critical role for Mtb cords in enhancing tolerance to antibiotic therapy. Wild-type Mtb that formed cords recovered and regrew faster than cording-deficient bacteria that formed clumps when perfusion of front-line anti-TB drugs such as isoniazid (INH) and bedaquiline (BDQ) through the vascular channel in the lung-chip model ceased. Incidentally, for both strains, regrowth in the lung-on-chip model was in sharp contrast to sterilization at the same antibiotic concentration in standard macrophage models of infection. The lung-on-chip is thus an improved model for the in vivo recalcitrance of Mtb to antibiotic clearance and a good platform to directly demonstrate how cord architectures, likely by virtue of the tight-packing of bacteria, have important therapeutic consequences.
By thinking of Mtb in infection as aggregates and not single bacteria, we can imagine new interactions with host proteins for known effectors of Mtb pathogenesis and a new paradigm in pathogenesis where forces from bacterial architectures affect host function. We obtain a better appreciation of the challenges for Mtb clearance via antibiotic therapy and demonstrated the strength of the lung-on-chip as a model system to study this. Furthermore, the agent-based model provides a new framework to understand how architectures where adhesion between bacteria is significant can form and behave as unconventional biofilms.
